Overview of Research
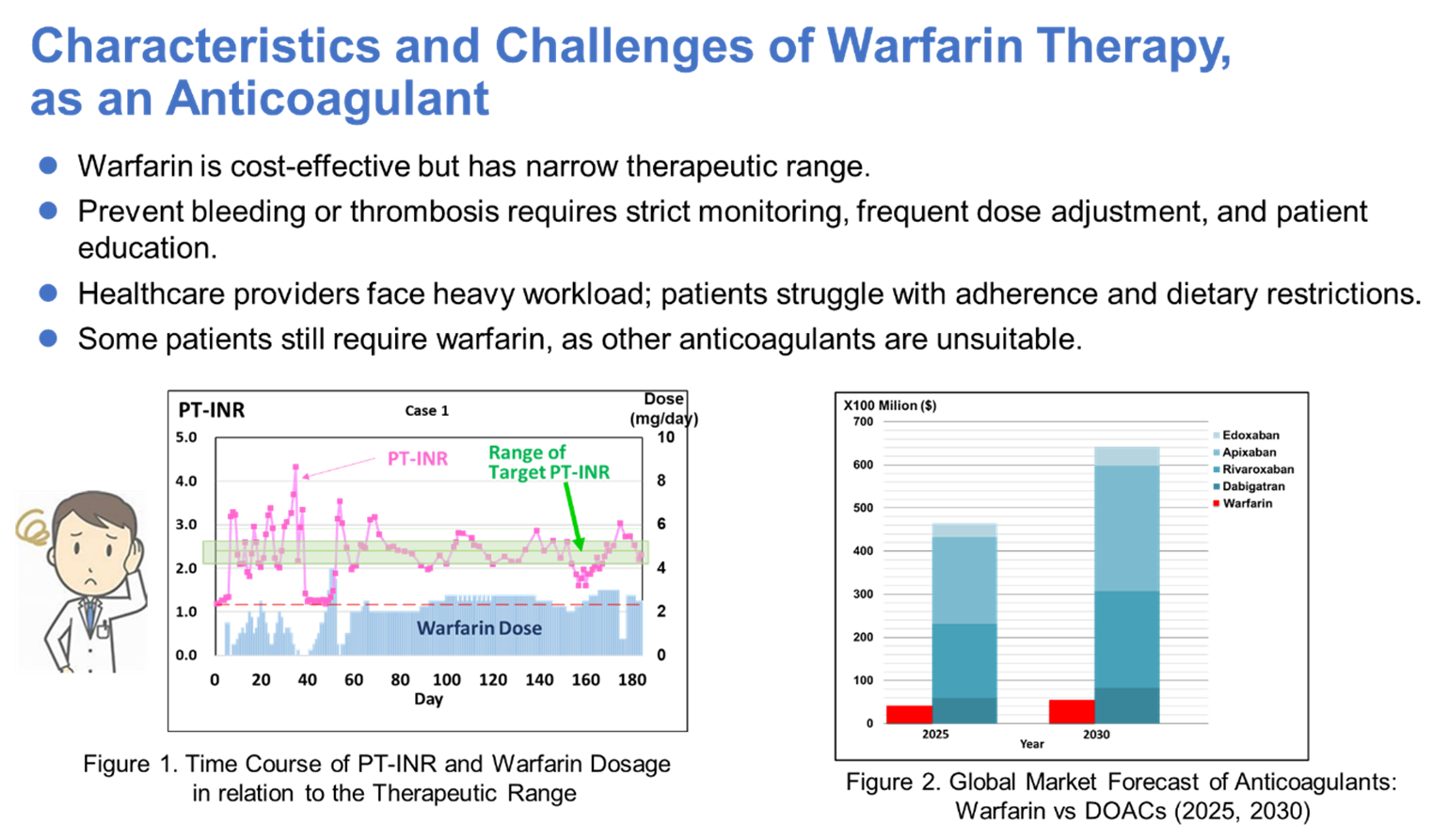
In this project, we aim to broaden the application of splice-switching oligonucleotides (SSOs) for rare genetic disorders by developing a novel design platform that enables efficient and precise exon skipping. This platform will expand the therapeutic scope of SSOs, facilitating the development of innovative RNA-targeted therapies for patients with currently unmet medical needs. As a proof of concept, we are targeting Duchenne muscular dystrophy(DMD), a disease with existing approved SSOs and the fastest path to expanding this approach to more patients. Using our platform, we have already identified candidate sequences with exceptionally high skipping efficiency, against multiple exons for which no effective therapies currently exist. In parallel, we are exploring strategies to improve the efficacy of existing SSOs and to overcome broader challenges such as delivery, aiming to establish next-generation RNA therapeutics with transformative clinical impact worldwide. By pushing the boundaries of SSO technology, we hope to unlock unprecedented therapeutic opportunities for patients long left without options.
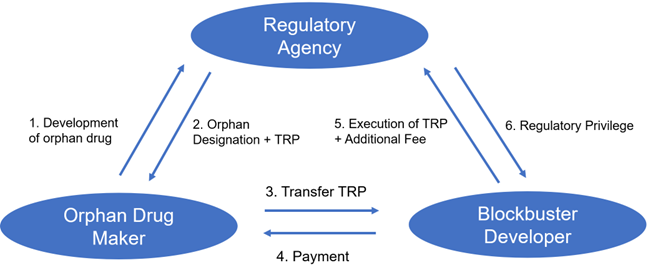
Figure1: Our strategy
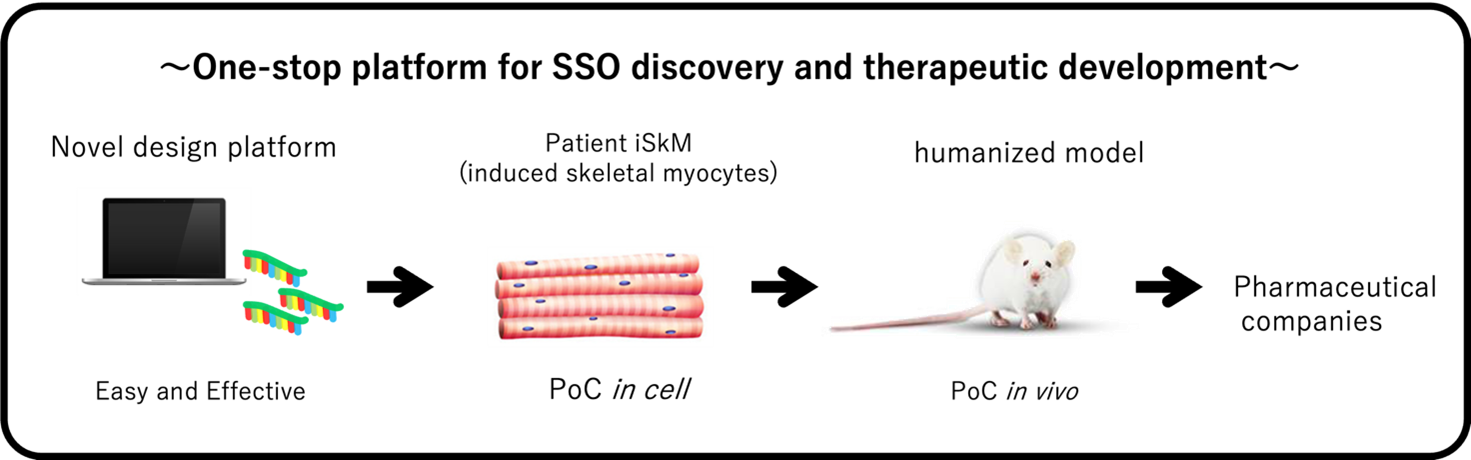
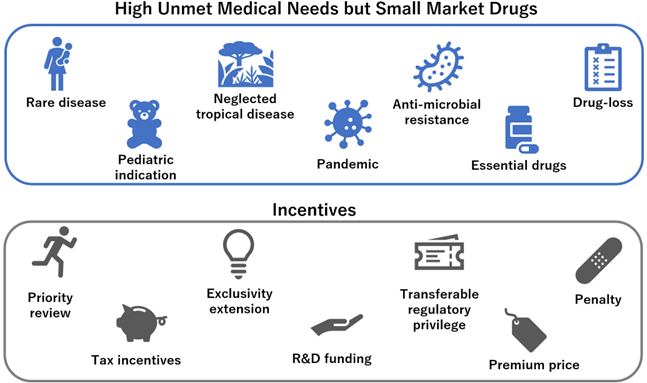
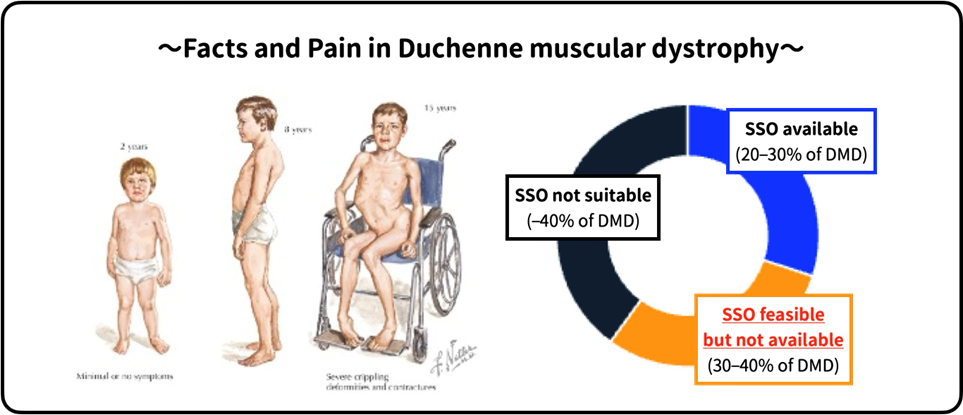
Figure2: Unmet needs for DMD
Profile
Dr.Awaya is a stem cell researcher, pediatric neurologist, and clinical geneticist at Kyoto University. His work bridges basic science and clinical application, focusing on RNA-targeted therapeutics such as small molecules and antisense oligonucleotides. Using iPSC-based disease models, he investigates rare genetic disorders to uncover mechanisms and develop therapies. Clinically, he specializes in rare pediatric genetic diseases, such as neuromuscular disorders, neurodegenerative disorders, and inborn errors of metabolism. As an educator, he teaches pediatric neurology and gross anatomy. Through his combined roles, he advances precision medicine and fosters academia–industry collaborations to deliver transformative treatments.